
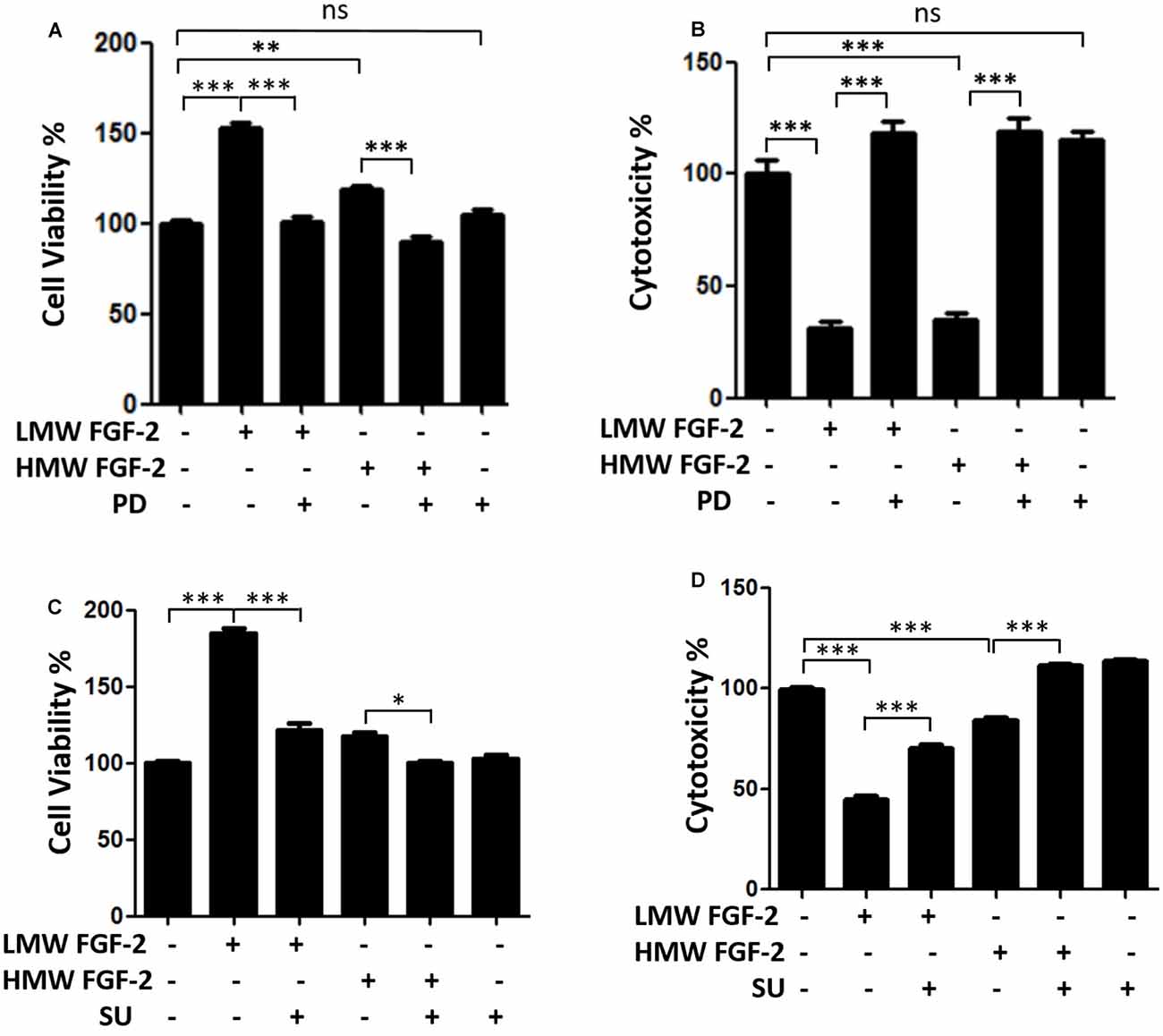
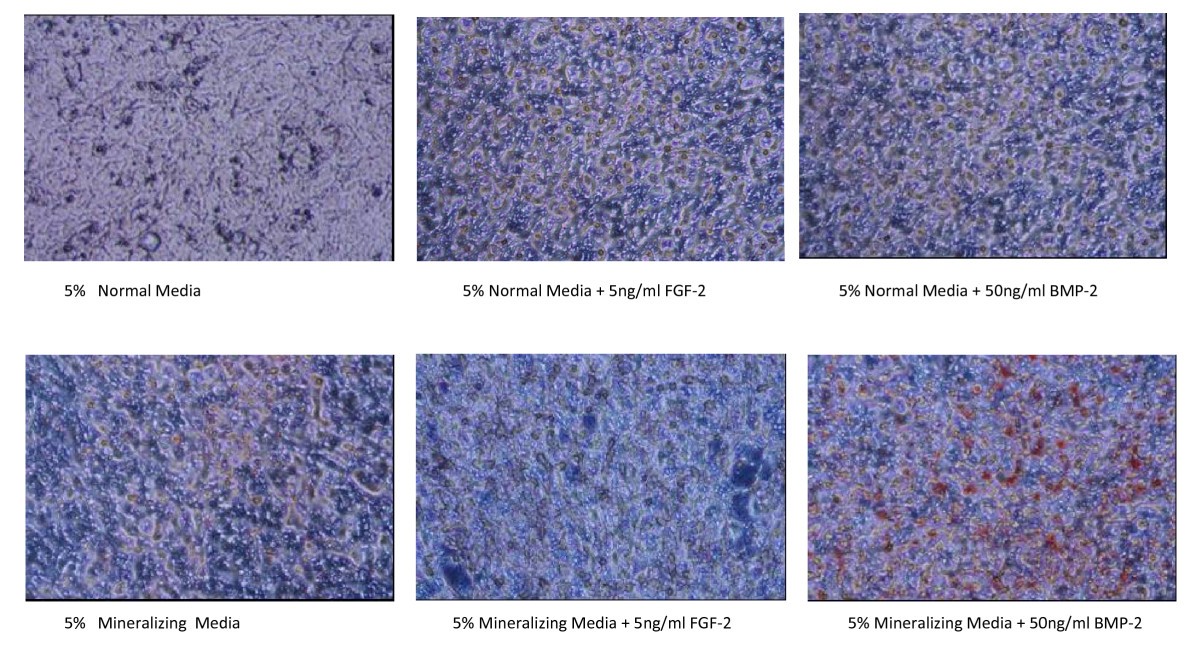
We hypothesized that other proteins from the bone marrow microenvironment would promote resistance to FLT3 inhibitors and tested known microenvironmental cytokines, growth factors, and soluble proteins for their ability to protect the FLT3-ITD AML cell line, MOLM14, from AC220 treatment. However, FL is likely not the only component of the bone marrow microenvironment that provides sanctuary to leukemia cells. FL ligand expression increases during therapy with FLT3 inhibitors, providing one potential explanation of why leukemia cells in the bone marrow are relatively more resistant to FLT3 inhibitors ( 14). Recently, the ligand for FLT3 (FL) was also found to promote resistance to FLT3 inhibitors ( 14). However, resistance also occurs in the absence of FLT3 mutations, although the nature of this resistance is less clear. In particular, mutations of the “gatekeeper” residue F691L and mutations in the activation loop (the region around D835) confer resistance to AC220 both in vitro and in vivo ( 12). Similar to resistance in CML, point mutations in FLT3 have been shown to decrease affinity of AC220 for its target, leading to clinical resistance ( 11–13). However, despite a higher response rate, the durability of AC220 response is still quite limited, and most patients develop resistance after a few months of therapy. Recently, quizartinib (AC220), a more potent FLT3 inhibitor, was developed and demonstrated a 50% composite complete remission rate in phase II clinical trials ( 10).

Early generation FLT3 inhibitors only achieved a transient reduction in peripheral blasts, whereas bone marrow blasts were less affected ( 8, 9). The success of tyrosine kinase inhibitors, such as imatinib, in chronic myeloid leukemia (CML) generated considerable enthusiasm for FLT3 inhibitors in AML. Overall, these results support a strategy of early combination therapy to target early survival signals from the bone marrow microenvironment, in particular FGF2, to improve the depth of response in FLT3-ITD AML. FLT3-ITD AML patients treated with AC220 developed increased FGF2 expression in marrow stromal cells, which peaked prior to overt clinical relapse and detection of resistance mutations. Removing FL or FGF2 from ligand-dependent resistant cultures transiently restored sensitivity to AC220, but accelerated acquisition of secondary resistance via reactivation of FLT3 and RAS/MAPK signaling. Conversely, FGF2 promoted resistance through activation of FGFR1 and downstream MAPK effectors these resistant cells responded synergistically to combinatorial inhibition of FGFR1 and FLT3. FL directly attenuated AC220 inhibition of FLT3, consistent with previous reports. In this study, we outline a two-step model of resistance whereby extrinsic microenvironmental proteins FLT3 ligand (FL) and fibroblast growth factor 2 (FGF2) protect FLT3-ITD+ MOLM14 cells from AC220, providing time for subsequent accumulation of ligand-independent resistance mechanisms. However, responses are not durable and resistance develops within months. Santa Cruz Biotechnology FGF-2 antibody (santa Cruz, sc-365106) was used in immunohistochemistry - frozen section on rat samples at 1:300 (fig 4).Potent FLT3 inhibitors, such as quizartinib (AC220), have shown promise in treating acute myeloid leukemia (AML) containing FLT3 internal tandem duplication (ITD) mutations. immunohistochemistry - frozen section rat 1:300 fig 4.Santa Cruz Biotechnology FGF-2 antibody (Santa Cruz, sc-271847) was used in western blot on rat samples at 1:1000 (fig 5). In order to study B-type natriuretic peptide knock-out females for arterial remodeling, Santa Cruz Biotechnology FGF-2 antibody (Santa Cruz, sc-365106) was used in immunohistochemistry - frozen section on rat samples (fig 2). immunohistochemistry - frozen section rat fig 2.Santa Cruz Biotechnology FGF-2 antibody (Santa Cruz, sc-74412) was used in western blot on human samples (fig 2). Santa Cruz Biotechnology FGF-2 antibody (Santa Cruz, sc-271847) was used in western blot on mouse samples at 1:1000 (fig 2e, 2f). Santa Cruz Biotechnology FGF-2 antibody (Santa Cruz Biotechnology, SC-365106) was used in western blot on mouse samples at 1:500 (fig 1d). Santa Cruz Biotechnology FGF-2 antibody (Santa Cruz, sc-365106) was used in western blot on mouse samples (fig 6a). Santa Cruz Biotechnology mouse monoclonal (G-2)


 0 kommentar(er)
0 kommentar(er)
